| Immunomodulatory mechanism :
|
Anti-obesity (leptin sensitizer, upregulates STAT3 pathway, AGRP (Agouti-related peptide), reduced PERK phosphorylation (lower ER stress), ALT, AST, inhibits NF-κb, HSP90); Anti-inflammatory (inhibits NF-κb, ikkα, ikkβ, downregulates TGF-β, upregulates IL-10, activates caspase-3, JNK, ERK, p38 MAPK pathways); Anti-cancer (increases iκb-α, p27, Bax via inhibition of proteasomes, regulates P13K/Akt/mtor pathway, inhibits HIF-1, topoisomerase II); Neuroprotective (inhibits ROS production, MHC-II expression, lipid peroxidation, regulates BACE-1, downregulates CD40, TNF-α, GFAP, inos); Anti-atherosclerotic (inhibits LOX-1, ROS production, downregulates P-selectin, glycoprotein iib/iiia, VEGF expression); Antioxidant (regulates HO-1, gsts, NADP(H), NQO1, Nrf2 pathway, reduces inos, NO); Anti-angiogenesis (inhibits VEGF induced Akt/mtor/p70s6 K signaling, HIF-1α, Hsp90, Akt/enos signaling, ICAM-1, VCAM-1, suppresses TLR4 mediated NF-kb activation, VEGFR-1, VEGFR-2)
|
| Description : |
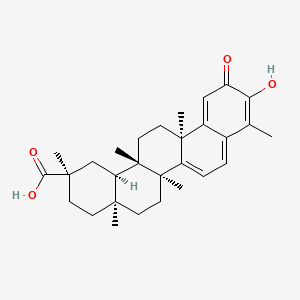
Celastrol, (9b,13a,14b,20a)-3-hydroxy-9,13-dimethyl-2-oxo-24,25,26-trinoroleana-1(10),3,5,7-tetraen-29-oic acid is a pentacyclic triterpenoid compound obtained from roots of thunder god vine. It has a pale brown to orange crystalline presence with high solubility in non polar compounds like ethanol, DMSO.
|