| ImmunCR Id : | ICR86 |
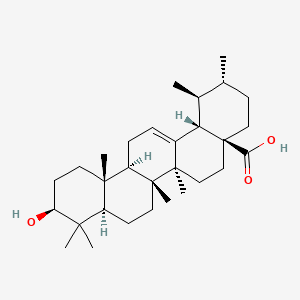
| Chemical Name : | Ursolic acid |
| Plant Source : | Salvia rosmarinus |
| Nutraceutical information : | Edible, Cornus mas (L.) (Red berries), Malus pumila (Mill.) (Rosaceae) (Apple) |
| Mode of administration : | Oral |
| Immunomodulatory mechanism : | Apoptosis (Releases cytochrome C, activates caspase-3, ROS, CHOP, JNK inactivates P13K/Akt, downregulates Bcl2, EGFR/MAPK pathway); Anti-metastasis (downregulates IL-1β, TNF-α, MMP-2, u-PA, inactivates NF-kβ, upregulates Iκβα inhibitors, suppresses CXCR4/CXCL12 receptor 4); Anti-angiogensis (suppresses VEGF, inos, HIF-1α, inhibits STAT3, SHH (sonic hedgehog) Akt/p70s6k signaling pathways); Antioxidant (upregulates GSH, CAT, SOD, gpx GAP43, downregulates lipid peroxidation, attenuates H2O2, MPP+, MDA, regulates NADPH oxidase-NF-kβ signaling pathway); Anti-inflammatory (downregulates IL-1β, TNF-α, IL-6, COX-2, inos, NF-AT, AP-1); Anti-aging (increase SIRT1, PGC-1α); Cardioprotective (reduces mir-21, p-ERK/ERK, increase camp level, acetylcholine) |
| Description : | Ursolic acid, 3β-hydroxy-urs-12-en-28-oic-acid occurs as a crystalline solid or needle like with low solubility in water but soluble in alcoholic naoh and hot glacial acetic acid. It is a pentacyclic triterpene with a C-30 isoprenoid and can exist as an aglycone of saponin or a free acid. |
| IUPAC Name | (1S,2R,4aS,6aR,6aS,6bR,8aR,10S,12aR,14bS)-10-hydroxy-1,2,6a,6b,9,9,12a-heptamethyl-2,3,4,5,6,6a,7,8,8a,10,11,12,13,14b-tetradecahydro-1H-picene-4a-carboxylic acid |
| SMILES | CC1CCC2(CCC3(C(=CCC4C3(CCC5C4(CCC(C5(C)C)O)C)C)C2C1C)C)C(=O)O |
| Formula | C30H48O3 |
| InchiKey | WCGUUGGRBIKTOS-UHFFFAOYSA-N |
| Kingdom | Organic compounds |
| Superclass | Lipids and lipid-like molecules |
| Class | Prenol lipids |
| Subclass | Triterpenoids |
| LogP | 6.492 |
| Molecular weight | 456.7 |
| Hydrogen Bond Acceptor | 3 |
| Hydrogen Bond Donor | 2 |
| Polar surface area | 58.931 |
| No. of rotatable bonds | 1 |
| Number of Aromatic Rings | 0 |
| Number of rings | 5 |
| Absorption level | Moderate |
| Solubility level | Very Soluble |
| Blood Brain Barrier | Undefined |
| Plasma protein binding | Binder |
| CYP2D6 inhibition | Non-Inhibitor |