| ImmunCR Id : | ICR83 |
| Chemical Name : | Paclitaxel |
| Plant Source : | Paclitaxel |
| Nutraceutical information : | --- |
| Mode of administration : | --- |
| Immunomodulatory mechanism : | Anti-cancer (G2/M phase cell cycle arrest, upregulates IL-10, CD4+, CD45 RO+, CD8+, CD45 Ro+, IFN-γ, downregulates Tregs) |
| Description : | Paclitaxel is a white cyrtalline powder which was first isolated in 1960 from Pacific yew (T. Brevifolia). It is a known taxane and widely studied for its potency against cancer because of its ability to stabilize microtubules. PTX is tricyclic diterpene, having a taxane complex ring with an amide function. The side chain at position C-13 adds to its cytotoxicity. The use of paclitaxel has been discontinued relating to the neurotoxic effects after infusion. |
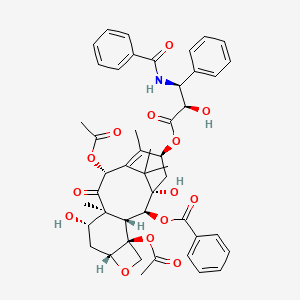
| IUPAC Name | [(1S,2S,3R,4S,7R,9S,10S,12R,15S)-4,12-diacetyloxy-15-[(2R,3S)-3-benzamido-2-hydroxy-3-phenylpropanoyl]oxy-1,9-dihydroxy-10,14,17,17-tetramethyl-11-oxo-6-oxatetracyclo[11.3.1.03,10.04,7]heptadec-13-en-2-yl] benzoate |
| SMILES | CC1=C2C(C(=O)C3(C(CC4C(C3C(C(C2(C)C)(CC1OC(=O)C(C(C5=CC=CC=C5)NC(=O)C6=CC=CC=C6)O)O)OC(=O)C7=CC=CC=C7)(CO4)OC(=O)C)O)C)OC(=O)C |
| Formula | C47H51NO14 |
| InchiKey | RCINICONZNJXQF-UHFFFAOYSA-N |
| Kingdom | Organic compounds |
| Superclass | Lipids and lipid-like molecules |
| Class | Prenol lipids |
| Subclass | Diterpenoids |
| LogP | 3.055 |
| Molecular weight | 853.906 |
| Hydrogen Bond Acceptor | 14 |
| Hydrogen Bond Donor | 4 |
| Polar surface area | 223.712 |
| No. of rotatable bonds | 14 |
| Number of Aromatic Rings | 3 |
| Number of rings | 7 |
| Absorption level | Very low |
| Solubility level | Very Soluble |
| Blood Brain Barrier | Undefined |
| Plasma protein binding | Binder |
| CYP2D6 inhibition | Non-Inhibitor |