| ImmunCR Id : | ICR65 |
| Chemical Name : | Artemisinin |
| Plant Source : | Artemisia annua L |
| Nutraceutical information : | --- |
| Mode of administration : | Oral, intramuscular, Rectal |
| Immunomodulatory mechanism : | Anti-cancer (inhibits NF-κb, Wnt/β-catenin pathway, downregulates VEGF (vascular endothelial growth factor), upregulates ROS, caspase 3, 9 and 8, Bak, Smac, AIF, reduced LMVD (lymphatic microvessel density)); Anti-proliferative (G1, G2/M cell cycle arrest, downregulate p-Akt, GSK3β (p-glycogen synthase kinase 3 beta), downregulates protein and mrna expression of PCNA(proliferating cell nuclear antigen)); Anti-inflammatory (downregulates ige, IL-10,4,5, IL-1β, TNF, ifnγ, histamine, upregulates Treg (regulatory T)) |
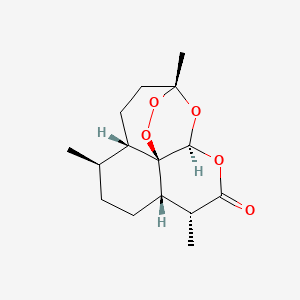
| Description : | Artemisinin, a sesquiterpene lactone having an endoperoxide moeity that reacts with heme acting against malaria parasites by forming free radicals. |
| IUPAC Name | (1R,4S,5R,8S,9R,12S,13R)-1,5,9-trimethyl-11,14,15,16-tetraoxatetracyclo[10.3.1.04,13.08,13]hexadecan-10-one |
| SMILES | CC1CCC2C(C(=O)OC3C24C1CCC(O3)(OO4)C)C |
| Formula | C15H22O5 |
| InchiKey | BLUAFEHZUWYNDE-UHFFFAOYSA-N |
| Kingdom | Organic compounds |
| Superclass | Lipids and lipid-like molecules |
| Class | Prenol lipids |
| Subclass | Terpene lactones |
| LogP | 1.998 |
| Molecular weight | 282.332 |
| Hydrogen Bond Acceptor | 5 |
| Hydrogen Bond Donor | 0 |
| Polar surface area | 53.021 |
| No. of rotatable bonds | 0 |
| Number of Aromatic Rings | 0 |
| Number of rings | 4 |
| Absorption level | Good |
| Solubility level | Very Soluble |
| Blood Brain Barrier | Medium |
| Plasma protein binding | Non-Binder |
| CYP2D6 inhibition | Non-Inhibitor |