| ImmunCR Id : | ICR59 |
| Chemical Name : | Caffeic acid |
| Plant Source : | Echinacea purpurea. |
| Nutraceutical information : | Edible, Apple, Coffee, Tea, Wine, Olive, Potato, Carrot, Blueberry, Cider. |
| Mode of administration : | Cream, Oral |
| Immunomodulatory mechanism : | Antioxidant (eliminates ROS, superoxide dismutase (SOD), chelates iron and copper ions, protects α-tocopherol); Photoprotection (inhibit collagenase matrix metalloproteinase-1 (MMP-1)activity, inhibit the nitric oxide produced by keratinocytes); Antimicrobial (inhibit E. Coli, Staphylococcus aureus, Bacillus cereus, Pseudomonas aeruginosa, Kokuria rhizophila, Candida albicans, Listeria monocytogenes growth); Anti-cancer (inhibits COX-2, IL-6, p38/MAPK activation, reduced IL-10 mrna expression, G2/M cell cycle arrest, reduced phosphorylation of JNK-1, downregulation of MMP-2, MMP-9 expression, inhibit nfκβ, downregulate p53 activation) |
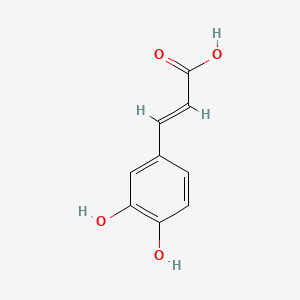
| Description : | 3,4-dihydroxycinnamic, commonly known as Caffeic acid is a phenolic compound found in plant kingdom synthesized through eendogenous shikimate pathway from glucose. It is a metabolite of hydroxycinnamate and phenylpropanoid. The structure of the caffeic acid is composed of the benzyl ring with a carboxyl group and OH at positions R2 and R3. |
| IUPAC Name | (E)-3-(3,4-dihydroxyphenyl)prop-2-enoic acid |
| SMILES | C1=CC(=C(C=C1/C=C/C(=O)O)O)O |
| Formula | C9H8O4 |
| InchiKey | QAIPRVGONGVQAS-DUXPYHPUSA-N |
| Kingdom | Organic compounds |
| Superclass | Phenylpropanoids and polyketides |
| Class | Cinnamic acids and derivatives |
| Subclass | Hydroxycinnamic acids and derivatives |
| LogP | 1.443 |
| Molecular weight | 180.157 |
| Hydrogen Bond Acceptor | 4 |
| Hydrogen Bond Donor | 3 |
| Polar surface area | 79.747 |
| No. of rotatable bonds | 2 |
| Number of Aromatic Rings | 1 |
| Number of rings | 1 |
| Absorption level | Good |
| Solubility level | Very Soluble |
| Blood Brain Barrier | Low |
| Plasma protein binding | Non-Binder |
| CYP2D6 inhibition | Non-Inhibitor |