| ImmunCR Id : | ICR55 |
| Chemical Name : | Kaempherol |
| Plant Source : | Carica papaya |
| Nutraceutical information : | Edible, Highest content in Onion leaves, Cauliflower, Broccoli, strawberry, Gooseberry yellow, Gooseberry red, Black Tea, Carrot, Brinjal |
| Mode of administration : | Oral |
| Immunomodulatory mechanism : | Anti-inflammatory (downregulates IL-6, IL-1β, IL-18, TNF-α, increases Nrf2-regulated genes protein and mrna expression, CRP (C-reactive protein), inhibits NF-κb, TLR4, COX1, COX2, nitric oxide synthesis); Anti-atherogenic (downregulates ICAM-1, E-selectin); Anti-cancer (arrests G2/M phase, DNA fragmentation at sub-G0 phase, upregulates Bax, p53, p21, downregulates PI3K/AKT pathways, Bcl2, PLK-1, pakt, pirs-1, pmek 1/2, CDK1, cathepsin D, E-cadherin, Snail, N-cadherin, Slug, MMP2, activates PARP, Caspase-3,7,9, MAPK cascades, cysteine proteases); Antioxidant (reduces ROS, inhibits xanthine oxidase, activates heme oxygenase-1, catalase, superoxide dismutase); Neuroprotective (upregulates ERK 1/2-camp-responsive element-binding protein (CREB) pathway, Na+/K+-atpase activity, inhibit NLRP3 inflammasone) |
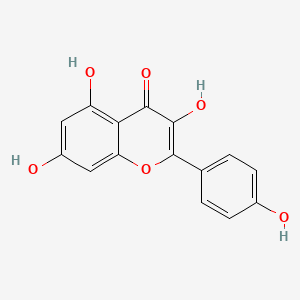
| Description : | Kaempferol‑3 or 3,5,7-trihydroxy-2-(4-hydroxyphenyl)-4H-chromen-4-one is a flavone with 4 hydroxy groups and yellow color presence, having high abundance in fruits and vegetables with many therapeutic possibilities. |
| IUPAC Name | 3,5,7-trihydroxy-2-(4-hydroxyphenyl)chromen-4-one |
| SMILES | C1=CC(=CC=C1C2=C(C(=O)C3=C(C=C(C=C3O2)O)O)O)O |
| Formula | C15H10O6 |
| InchiKey | IYRMWMYZSQPJKC-UHFFFAOYSA-N |
| Kingdom | Organic compounds |
| Superclass | Phenylpropanoids and polyketides |
| Class | Flavonoids |
| Subclass | Flavones |
| LogP | 1.872 |
| Molecular weight | 286.236 |
| Hydrogen Bond Acceptor | 6 |
| Hydrogen Bond Donor | 4 |
| Polar surface area | 109.492 |
| No. of rotatable bonds | 1 |
| Number of Aromatic Rings | 2 |
| Number of rings | 3 |
| Absorption level | Good |
| Solubility level | Very Soluble |
| Blood Brain Barrier | Low |
| Plasma protein binding | Non-Binder |
| CYP2D6 inhibition | Non-Inhibitor |