| ImmunCR Id : | ICR50 |
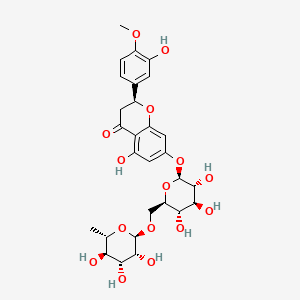
| Chemical Name : | Hesperidin |
| Plant Source : | Citrus sps. |
| Nutraceutical information : | Edible, high content in lime, lemon, orange, tangerines, clementines, mandarins |
| Mode of administration : | Oral, I.V |
| Immunomodulatory mechanism : | Anti-tumor (pro-apoptotic: suppresses phosphatidylinositol-4,5-bisphosphate 3-kinase subunit/threonine kinase/AKT Serine/ inhibit kappa light polypeptide gene enhancer in B-cells (PI3K/Akt/IKK) signalling pathway; breast cancer: phosphatidyl-serine externalization, activate caspase-7, 9, releases cytochrome c, increase Bax:Bcl-2 ratio; Hepatocellular carcinoma: ROS generation, high ATP and calcium levels; Lung cancer: upregulating expression of p53, p21; reduced expression of COX-2, TNF-α, NF-κb); Anti-angiogenic (target matrix metalloproteinases (MMP), vascular endothelial growth factor (VEGF), basic fibroblast growth factor (bfgf), blocks AKT, mtor signal pathway); Anti-inflammatory (reduced level of VCAM-1, , MMP-2, IL-4, MMP-9, COX-2, IL-6, inos, PGE2 (prostaglandin E2), NO2); Anti-oxidant (scavenge ROS, RNS, hydroxyl) |
| Description : | Hesp or Hesperetin 7-rutinoside is a bioactive compound of flavanone family obtained by alkaline hydrolysis of phlorogucinol and hesperentic acid, lacking double bond at position 2, 3 in the C ring, differentiating it from other flavones, isoflavones, flavanol. |
| IUPAC Name | (2S)-5-hydroxy-2-(3-hydroxy-4-methoxyphenyl)-7-[(2S,3R,4S,5S,6R)-3,4,5-trihydroxy-6-[[(2R,3R,4R,5R,6S)-3,4,5-trihydroxy-6-methyloxan-2-yl]oxymethyl]oxan-2-yl]oxy-2,3-dihydrochromen-4-one |
| SMILES | CC1C(C(C(C(O1)OCC2C(C(C(C(O2)OC3=CC(=C4C(=O)CC(OC4=C3)C5=CC(=C(C=C5)OC)O)O)O)O)O)O)O)O |
| Formula | C28H34O15 |
| InchiKey | QUQPHWDTPGMPEX-UHFFFAOYSA-N |
| Kingdom | Organic compounds |
| Superclass | Phenylpropanoids and polyketides |
| Class | Flavonoids |
| Subclass | Flavonoid glycosides |
| LogP | -0.431 |
| Molecular weight | 610.561 |
| Hydrogen Bond Acceptor | 15 |
| Hydrogen Bond Donor | 8 |
| Polar surface area | 237.405 |
| No. of rotatable bonds | 7 |
| Number of Aromatic Rings | 2 |
| Number of rings | 5 |
| Absorption level | Very low |
| Solubility level | Very Soluble |
| Blood Brain Barrier | Undefined |
| Plasma protein binding | Non-Binder |
| CYP2D6 inhibition | Non-Inhibitor |