| ImmunCR Id : | ICR5 |
| Chemical Name : | Quercetin |
| Plant Source : | Mango seed |
| Nutraceutical information : | Edible, apples, berries, Brassica vegetables, capers, grapes, onions, shallots, tea, tomatoes, nuts. |
| Mode of administration : | Oral |
| Immunomodulatory mechanism : | Antioxidant (reduced GSH concentrations, glutathione reductase activity); Anaphylatic reaction (decreased immunoglobulin E response, plasma histamine); Cardioprotective (inhibits platelet aggregation and thrombus formation); Diabetes (inhibits Aldose reductase); Anti-inflammatory (inhibits COX (cyclooxygenase), LOX (lipoxygenase), LPS-induced IL-8 and TNF-α, NO production, and (NOS) expression, tyrosine phosphorylation of JAK2, TYK2, STAT3, and STAT4); Antinociceptive (inhibits pronociceptive cytokine production, free radical generation, and influence L-arginine-nitric oxide, serotonin, gabaergic); Anxiety and Depression (decrease brain corticotropin-releasing factor (CRF) expression, increases plasma corticosterone and adrenocorticotropic hormone); Sleep (activates GABA(A) receptors); Anti-cancer (modulation of MEK/ERK and Nrf2/keap1 transduction signalling pathways, inhibit the tyrosine kinase activity) |
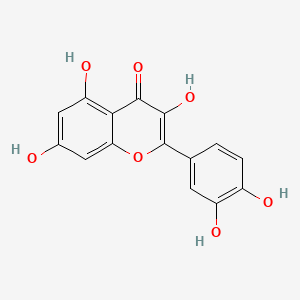
| Description : | As per nomencleature Quercetin is known as 3,3',4',5,7-pentahydroxyflavanone belonging to the flavanone class with hydroxyl group at 3, 5, 7, 3', and 4' positions, it is citron yellow in color, showing solubility in alcohol and lipids but not water but partial solubility in hot water. |
| IUPAC Name | 2-(3,4-dihydroxyphenyl)-3,5,7-trihydroxychromen-4-one |
| SMILES | C1=CC(=C(C=C1C2=C(C(=O)C3=C(C=C(C=C3O2)O)O)O)O)O |
| Formula | C15H10O7 |
| InchiKey | REFJWTPEDVJJIY-UHFFFAOYSA-N |
| Kingdom | Organic compounds |
| Superclass | Phenylpropanoids and polyketides |
| Class | Flavonoids |
| Subclass | Flavones |
| LogP | 1.63 |
| Molecular weight | 302.236 |
| Hydrogen Bond Acceptor | 7 |
| Hydrogen Bond Donor | 5 |
| Polar surface area | 130.308 |
| No. of rotatable bonds | 1 |
| Number of Aromatic Rings | 2 |
| Number of rings | 3 |
| Absorption level | Moderate |
| Solubility level | Very Soluble |
| Blood Brain Barrier | Undefined |
| Plasma protein binding | Non-Binder |
| CYP2D6 inhibition | Non-Inhibitor |