| ImmunCR Id : | ICR44 |
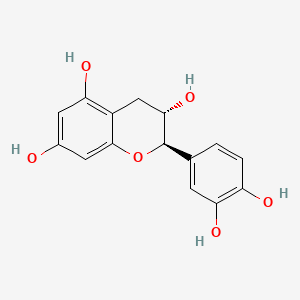
| Chemical Name : | Catechin |
| Plant Source : | Pericarpium |
| Nutraceutical information : | Edible, apples, blueberries, gooseberries, grape seeds, kiwi, strawberries, green tea, red wine, beer, cacao liquor, chocolate, cocoa |
| Mode of administration : | --- |
| Immunomodulatory mechanism : | Antioxidant (electron/proton donating capacity in (1)-catechin and its radical scavenging antioxidant activity influencing the deprotonation of the catechol group; inhibiting major enzymes involved in lipid biosynthesis, reducing intestinal lipid absorption, activates endothelial nitric oxide, regulating of vascular tone), ROS scavenger, anti inflammatory (significantly decreased the levels of nitric oxide (NO), TNF-α, and IL-1β mrna; prevention of atherosclerosis progression due to vascular anti-inflammatory action, and the inhibition of vascular cell growth factors involved in atherosclerosis; decreased production of malondialdehyde activity (MDA) of the platelets), , protects against ulcerative colitis, neuroprotective; receptor tyrosine kinase inhibition; Inhibition of thrombogenesis by suppressing platelet adhesion. |
| Description : | A flavan-3-ol, secondary metabolite, possessing two benzene rings, a C ring with hydroxyl on carbon 3. |
| IUPAC Name | (2R,3S)-2-(3,4-dihydroxyphenyl)-3,4-dihydro-2H-chromene-3,5,7-triol |
| SMILES | C1[C@@H]([C@H](OC2=CC(=CC(=C21)O)O)C3=CC(=C(C=C3)O)O)O |
| Formula | C15H14O6 |
| InchiKey | PFTAWBLQPZVEMU-DZGCQCFKSA-N |
| Kingdom | Organic compounds |
| Superclass | Phenylpropanoids and polyketides |
| Class | Flavonoids |
| Subclass | Flavans |
| LogP | 2.021 |
| Molecular weight | 290.268 |
| Hydrogen Bond Acceptor | 6 |
| Hydrogen Bond Donor | 5 |
| Polar surface area | 113.007 |
| No. of rotatable bonds | 1 |
| Number of Aromatic Rings | 2 |
| Number of rings | 3 |
| Absorption level | Good |
| Solubility level | Very Soluble |
| Blood Brain Barrier | Undefined |
| Plasma protein binding | Non-Binder |
| CYP2D6 inhibition | Non-Inhibitor |