| ImmunCR Id : | ICR30 |
| Chemical Name : | Cis-resveratrol |
| Plant Source : | Vitis palmata |
| Nutraceutical information : | Edible, high content in grapes, wine. |
| Mode of administration : | Oral, Injection |
| Immunomodulatory mechanism : | Antiinflammatory, anticancer, antidiabetes, neuroprotection, cardioprotection, and energy homeostasis, blocked the expression of genes related to the REL/NF-nb/inb family, adhesion molecules and acute-phase proteins. In inflammatory peritoneal macrophages stimulated with lipopolysaccharide (LPS) and gamma interferon (IFN-g). Down-regulated the nuclear factor of kappa light chain gene enhancer in B-cells 1 (nfnbl1) gene product p105 and up-regulated the nuclear factor of kappa light chain gene enhancer in B-cells inhibitor alpha (inba) gene. Inhibited intercellular adhesion molecule1 (ICAM-1) gene expression and the transmembrane receptors RIP (receptor TNFRSF) and TLR3 (toll-like receptor 7). Inhibited transcription of Scya2 (chemokine MCP-1), the chemokine RANTES (regulated on activation, normal T cell expressed and secreted), pro-inflammatory cytokines that attract monocyte–granulocyte cells such as M-CSF (colony-stimulating factor 1), GM-CSF (colony-stimulating factor 2) and G-CSF (colony-stimulating factor 3), the cytokine tumor growth factor beta (TGF-h) and the extracellular ligand IL-1a. In contrast, c-RESV stimulated transcription of the pro-inflammatory cytokines IL-6 and tumor necrosis factor alpha (TNF-a), the extracellular ligand IL-1h, and the IFN regulatory factor (IRF)-1. Blocks radical oxygen species (ROS) production by inflammatory macrophages (by inhibition of NADH/NADPH oxidase activity) and inhibits the production of the proinflammatory mediators NO and PGE2 (at least in part by inhibition of NOS-2 and COX-2 gene and protein expression). |
| Description : | The cis isomer of resveratol is unstable in this form and is turned into trans state in the grapes, resveratol is a specific inhibitor of COX-1 and a potent antioxidant with a minor presence in wine |
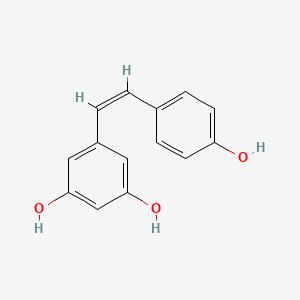
| IUPAC Name | 5-[(Z)-2-(4-hydroxyphenyl)ethenyl]benzene-1,3-diol |
| SMILES | C1=CC(=CC=C1/C=C\C2=CC(=CC(=C2)O)O)O |
| Formula | C14H12O3 |
| InchiKey | LUKBXSAWLPMMSZ-UPHRSURJSA-N |
| Kingdom | Organic compounds |
| Superclass | Phenylpropanoids and polyketides |
| Class | Stilbenes |
| Subclass | --- |
| LogP | 3.09 |
| Molecular weight | 228.243 |
| Hydrogen Bond Acceptor | 3 |
| Hydrogen Bond Donor | 3 |
| Polar surface area | 62.446 |
| No. of rotatable bonds | 2 |
| Number of Aromatic Rings | 2 |
| Number of rings | 2 |
| Absorption level | Good |
| Solubility level | Very Soluble |
| Blood Brain Barrier | Medium |
| Plasma protein binding | Binder |
| CYP2D6 inhibition | Non-Inhibitor |