| ImmunCR Id : | ICR3 |
| Chemical Name : | Caffeic acid |
| Plant Source : | Echinacea purpurea |
| Nutraceutical information : | Edible, Blueberries, coffee, apple, cider, oils (soybean, grape seed, sunflower, sesame, corn, etc.), tea |
| Mode of administration : | Oral |
| Immunomodulatory mechanism : | Antioxidant (inhibit COX-2, photoprotective effect through glutathione metabolism, DPPH radical scavenging, ROS reduction, inhibit lipid peroxidation, protect against LDL oxidation); Antiviral (inhibit hbsag, hbeag secretion, DHBV-DNA); Anti-cancer (antiproliferation effect against hela cell line, cellular entrapment in the G2/M phase, reduced mrna expression of IL-10); Anti-HIV (HIV integrase inhibition); Antimicrobial (inhibits proliferation of E. Coli, Staphylococcus aureus, Bacillus cereus, Listeria monocytogenes) |
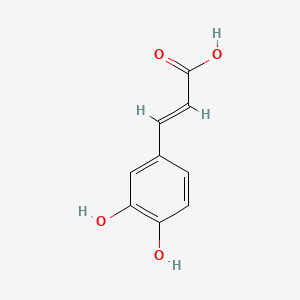
| Description : | Caffeic acid or 3,4-dihydroxycinnamic acid is a hydroxycinnamate and phenylpropanoid metabolite and widely distributed in nature with many health benefits like anti-cancer, antioxidant and can be extracted from multiple plants. It is a catechol derivative and a phenolic acid. |
| IUPAC Name | (E)-3-(3,4-dihydroxyphenyl)prop-2-enoic acid |
| SMILES | C1=CC(=C(C=C1/C=C/C(=O)O)O)O |
| Formula | C9H8O4 |
| InchiKey | QAIPRVGONGVQAS-DUXPYHPUSA-N |
| Kingdom | Organic compounds |
| Superclass | Phenylpropanoids and polyketides |
| Class | Cinnamic acids and derivatives |
| Subclass | Hydroxycinnamic acids and derivatives |
| LogP | 1.443 |
| Molecular weight | 180.157 |
| Hydrogen Bond Acceptor | 4 |
| Hydrogen Bond Donor | 3 |
| Polar surface area | 79.747 |
| No. of rotatable bonds | 2 |
| Number of Aromatic Rings | 1 |
| Number of rings | 1 |
| Absorption level | Good |
| Solubility level | Very Soluble |
| Blood Brain Barrier | Low |
| Plasma protein binding | Non-Binder |
| CYP2D6 inhibition | Non-Inhibitor |