| ImmunCR Id : | ICR28 |
| Chemical Name : | Epigallocatechin-3-gallate (EGCG) |
| Plant Source : | Camellia sinensis |
| Nutraceutical information : | Edible, Green tea with high antioxidant properties |
| Mode of administration : | --- |
| Immunomodulatory mechanism : | Strong expanding beneficial effects in studies of diabetes, Parkinson's disease, Alzheimer's disease, stroke, inflammation, obesity, and cancer. Exerts its antioxidant activity via decreasing NO and Malondialdehyde (MDA), and increasing Superoxide dismutase (SOD), and ameliorates mucosal inflammation by inhibiting the production of TNF-α, IFN-γ, and NF-κb. EGCG reduces experimental colon injury by inhibiting macrophage chemotaxis toward N-formyl-l-leucyl-l-phenylalanine, thereby suppressing the mast cells and macrophage activities |
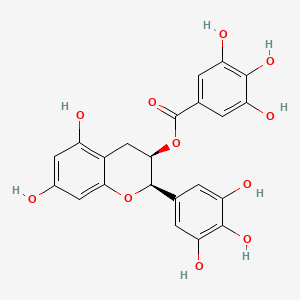
| Description : | EGCG a polyphenol is an ester formed by condensation of gallic acid with he (3R)-hydroxy group of (-)-epigallocatechin |
| IUPAC Name | [(2R,3R)-5,7-dihydroxy-2-(3,4,5-trihydroxyphenyl)-3,4-dihydro-2H-chromen-3-yl] 3,4,5-trihydroxybenzoate |
| SMILES | C1C(C(OC2=CC(=CC(=C21)O)O)C3=CC(=C(C(=C3)O)O)O)OC(=O)C4=CC(=C(C(=C4)O)O)O |
| Formula | C22H18O11 |
| InchiKey | WMBWREPUVVBILR-UHFFFAOYSA-N |
| Kingdom | Organic compounds |
| Superclass | Phenylpropanoids and polyketides |
| Class | Flavonoids |
| Subclass | Flavans |
| LogP | 3.097 |
| Molecular weight | 458.372 |
| Hydrogen Bond Acceptor | 11 |
| Hydrogen Bond Donor | 8 |
| Polar surface area | 201.684 |
| No. of rotatable bonds | 4 |
| Number of Aromatic Rings | 3 |
| Number of rings | 4 |
| Absorption level | Very low |
| Solubility level | Very Soluble |
| Blood Brain Barrier | Undefined |
| Plasma protein binding | Non-Binder |
| CYP2D6 inhibition | Non-Inhibitor |