| ImmunCR Id : | ICR143 |
| Chemical Name : | Dehydroepiandrosterone |
| Plant Source : | Adrenal gland |
| Nutraceutical information : | Used as a nutritional supplement for anti-aging, arthiritis |
| Mode of administration : | Capsule |
| Immunomodulatory mechanism : | DHEA is very effective at blunting both Th-1 and Th-2 immunological responses, and further suppresses expression of various proinflammatory cytokines |
| Description : | Prasterone is a synthetic form of dehydroepiandrosterone with potential chemopreventive activity. Produced endogenously, dehydroepiandrosterone (DHEA) is an intermediate in the conversion of cholesterol to androgens and estrogens. Although the mechanisms of action of exogenously administered DHEA have not been fully illuminated, they may result in both direct and indirect physiologic effects. Direct effects include GABA-a receptor complex and NMDA receptor modulation, and enhanced pancreatic beta cell insulin secretion and antiglucocorticoid activities. (NCI04) |
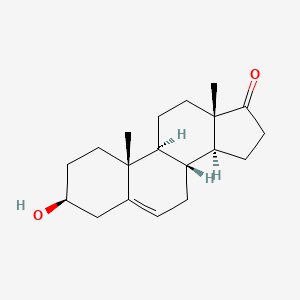
| IUPAC Name | (3S,8R,9S,10R,13S,14S)-3-hydroxy-10,13-dimethyl-1,2,3,4,7,8,9,11,12,14,15,16-dodecahydrocyclopenta[a]phenanthren-17-one |
| SMILES | C[C@]12CC[C@H]3[C@H]([C@@H]1CCC2=O)CC=C4[C@@]3(CC[C@@H](C4)O)C |
| Formula | C19H28O2 |
| InchiKey | FMGSKLZLMKYGDP-USOAJAOKSA-N |
| Kingdom | Organic compounds |
| Superclass | Lipids and lipid-like molecules |
| Class | Steroids and steroid derivatives |
| Subclass | Androstane steroids |
| LogP | 3.338 |
| Molecular weight | 288.424 |
| Hydrogen Bond Acceptor | 2 |
| Hydrogen Bond Donor | 1 |
| Polar surface area | 38.116 |
| No. of rotatable bonds | 0 |
| Number of Aromatic Rings | 0 |
| Number of rings | 4 |
| Absorption level | Good |
| Solubility level | Very Soluble |
| Blood Brain Barrier | High |
| Plasma protein binding | Binder |
| CYP2D6 inhibition | Non-Inhibitor |