| ImmunCR Id : | ICR142 |
| Chemical Name : | Chondroitin sulfate |
| Plant Source : | Cow shark |
| Nutraceutical information : | Used as a nutritional supplement for joint pain |
| Mode of administration : | Oral |
| Immunomodulatory mechanism : | By inhibiting nuclear translocation of NF-κB and subsequent production of pro-inflammatory cytokines, and COX-2 and PLA2 expression and activity (Fig. 3), CS might potentially be of interest for the treatment of many inflammatory and autoimmune diseases |
| Description : | Chondroitin sulfate is a glycosaminoglycan considered as a symptomatic slow-acting drug for osteoarthritis (SYSADOA). The SYSADOA status suggested a pain relief and increased joint mobility after a relative long regular administration, as well as a long-lasting effect after the end of the treatment. Chondroitin sulfate is composed of alternating 1,3-N-acetyl-?-d-galactosamine and 1,4-?-d-glucuronic acid units which bear 4-O- and/or 6-O-sulfations at the N-acetylgalactosamine units disposed of in specific patterns. Depending on the predominating disaccharide unit, it will present different biological activities. Chondroitin sulfate is sold as an OTC dietary supplement in North America and it is a prescription drug under the EMA in Europe. |
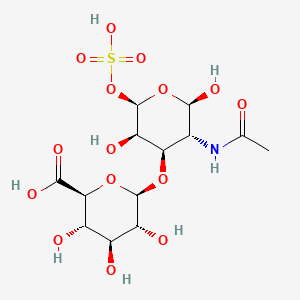
| IUPAC Name | (2S,3S,4S,5R,6R)-6-[(2R,3R,4R,5R,6R)-3-acetamido-2,5-dihydroxy-6-sulfooxyoxan-4-yl]oxy-3,4,5-trihydroxyoxane-2-carboxylic acid |
| SMILES | CC(=O)N[C@@H]1[C@H]([C@H]([C@H](O[C@H]1O)OS(=O)(=O)O)O)O[C@H]2[C@@H]([C@H]([C@@H]([C@H](O2)C(=O)O)O)O)O |
| Formula | C13H21NO15S |
| InchiKey | KXKPYJOVDUMHGS-OSRGNVMNSA-N |
| Kingdom | Organic compounds |
| Superclass | Organic oxygen compounds |
| Class | Organooxygen compounds |
| Subclass | Carbohydrates and carbohydrate conjugates |
| LogP | -4.44 |
| Molecular weight | 463.369 |
| Hydrogen Bond Acceptor | 15 |
| Hydrogen Bond Donor | 8 |
| Polar surface area | 263.442 |
| No. of rotatable bonds | 6 |
| Number of Aromatic Rings | 0 |
| Number of rings | 2 |
| Absorption level | Very low |
| Solubility level | Very Soluble |
| Blood Brain Barrier | Undefined |
| Plasma protein binding | Non-Binder |
| CYP2D6 inhibition | Non-Inhibitor |