| ImmunCR Id : | ICR140 |
| Chemical Name : | Campestanol |
| Plant Source : | Many vegetables, fruits, nuts, and seeds |
| Nutraceutical information : | --- |
| Mode of administration : | --- |
| Immunomodulatory mechanism : | phytosterols are important in host systems and exert antitumor effects by improving the immune system’s identification of cancer, affecting hormone-dependent (hormone-dependent) endocrine tumor growth, and regulating sterol biosynthesis |
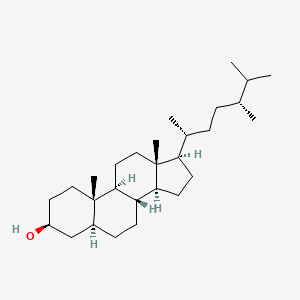
| Description : | Campestanol is a 3beta-sterol and a C28-steroid. It derives from a hydride of a 5alpha-campestane. |
| IUPAC Name | (3S,5S,8R,9S,10S,13R,14S,17R)-17-[(2R,5R)-5,6-dimethylheptan-2-yl]-10,13-dimethyl-2,3,4,5,6,7,8,9,11,12,14,15,16,17-tetradecahydro-1H-cyclopenta[a]phenanthren-3-ol |
| SMILES | C[C@H](CC[C@@H](C)C(C)C)[C@H]1CC[C@@H]2[C@@]1(CC[C@H]3[C@H]2CC[C@@H]4[C@@]3(CC[C@@H](C4)O)C)C |
| Formula | C28H50O |
| InchiKey | ARYTXMNEANMLMU-ATEDBJNTSA-N |
| Kingdom | Organic compounds |
| Superclass | Lipids and lipid-like molecules |
| Class | Steroids and steroid derivatives |
| Subclass | Ergostane steroids |
| LogP | 7.877 |
| Molecular weight | 402.696 |
| Hydrogen Bond Acceptor | 1 |
| Hydrogen Bond Donor | 1 |
| Polar surface area | 20.815 |
| No. of rotatable bonds | 5 |
| Number of Aromatic Rings | 0 |
| Number of rings | 4 |
| Absorption level | Very low |
| Solubility level | Very Soluble |
| Blood Brain Barrier | Undefined |
| Plasma protein binding | Binder |
| CYP2D6 inhibition | Non-Inhibitor |