| ImmunCR Id : | ICR138 |
| Chemical Name : | Astaxanthin |
| Plant Source : | Algae, yeast, salmon, trout, krill, shrimp and crayfish |
| Nutraceutical information : | Used as a dieatry supplement |
| Mode of administration : | Tablet, Liquid |
| Immunomodulatory mechanism : | Astaxanthin modulates the production of T helper 1 cytokines, such as IL-2, as well as IFN-γ, without causing significant cytotoxic effects in primary cultured lymphocytes. In addition, astaxanthin enhanced LPS- induced immune responses by stimulating production of cytokines, as well as enhancing Con A-induced IL-2 production |
| Description : | Astaxanthin is a natural and synthetic xanthophyll and nonprovitamin A carotenoid, with potential antioxidant, anti-inflammatory and antineoplastic activities. Upon administration, astaxanthin may act as an antioxidant and reduce oxidative stress, thereby preventing protein and lipid oxidation and DNA damage. By decreasing the production of reactive oxygen species (ROS) and free radicals, it may also prevent ROS-induced activation of nuclear factor-kappa B (NF-kB) transcription factor and the production of inflammatory cytokines such as interleukin-1beta (IL-1b), IL-6 and tumor necrosis factor-alpha (TNF-a). In addition, astaxanthin may inhibit cyclooxygenase-1 (COX-1) and nitric oxide (NO) activities, thereby reducing inflammation. Oxidative stress and inflammation play key roles in the pathogenesis of many diseases, including cardiovascular, neurological, autoimmune and neoplastic diseases. |
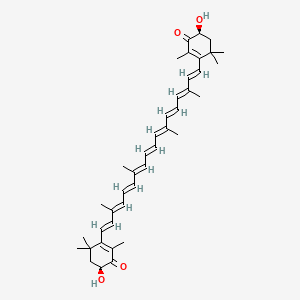
| IUPAC Name | (6S)-6-hydroxy-3-[(1E,3E,5E,7E,9E,11E,13E,15E,17E)-18-[(4S)-4-hydroxy-2,6,6-trimethyl-3-oxocyclohexen-1-yl]-3,7,12,16-tetramethyloctadeca-1,3,5,7,9,11,13,15,17-nonaenyl]-2,4,4-trimethylcyclohex-2-en-1-one |
| SMILES | CC1=C(C(C[C@@H](C1=O)O)(C)C)/C=C/C(=C/C=C/C(=C/C=C/C=C(/C=C/C=C(/C=C/C2=C(C(=O)[C@H](CC2(C)C)O)C)C)C)/C)/C |
| Formula | C40H52O4 |
| InchiKey | MQZIGYBFDRPAKN-UWFIBFSHSA-N |
| Kingdom | Organic compounds |
| Superclass | Lipids and lipid-like molecules |
| Class | Prenol lipids |
| Subclass | Tetraterpenoids |
| LogP | 8.421 |
| Molecular weight | 596.838 |
| Hydrogen Bond Acceptor | 4 |
| Hydrogen Bond Donor | 2 |
| Polar surface area | 76.232 |
| No. of rotatable bonds | 10 |
| Number of Aromatic Rings | 0 |
| Number of rings | 2 |
| Absorption level | Very low |
| Solubility level | Very Soluble |
| Blood Brain Barrier | Undefined |
| Plasma protein binding | Binder |
| CYP2D6 inhibition | Non-Inhibitor |