| ImmunCR Id : | ICR121 |
| Chemical Name : | Thymoquinone |
| Plant Source : | Black cumin |
| Nutraceutical information : | Black seed oil-containing capsules available as food or food supplements |
| Mode of administration : | Oral ingestion |
| Immunomodulatory mechanism : | TQ also reduced LPS-induced proinflammatory cytokines such as interleukins (ILs) and TNF-α. In addition, TQ showed the immunomodulatory role in cellular and humoral immunity. In autoimmune disease such as diabetes and experimental allergic encephalomyelitis, TQ reduced the levels of T-, B-lymphocytes markers, innate cell marker, and islet cell antibodies and also attenuated most of the pathological changes in pancreas in type 1 diabetes |
| Description : | Thymoquinone is a member of the class of 1,4-benzoquinones that is 1,4-bezoquinone in which the hydrogens at positions 2 and 5 are replaced by methyl and isopropyl groups, respectively. It is a natural compound isolated from Nigella sativa which has demonstrated promising chemotherapeutic activity. It has a role as an anti-inflammatory agent, an antioxidant, an adjuvant, an antineoplastic agent, a cardioprotective agent, an antidepressant and a plant metabolite. |
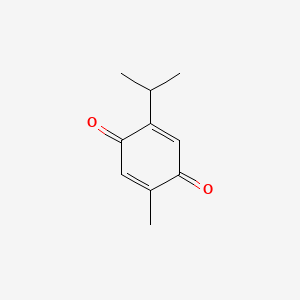
| IUPAC Name | 2-methyl-5-propan-2-ylcyclohexa-2,5-diene-1,4-dione |
| SMILES | CC1=CC(=O)C(=CC1=O)C(C)C |
| Formula | C10H12O2 |
| InchiKey | KEQHJBNSCLWCAE-UHFFFAOYSA-N |
| Kingdom | Organic compounds |
| Superclass | Organic oxygen compounds |
| Class | Organooxygen compounds |
| Subclass | Carbonyl compounds |
| LogP | 2.288 |
| Molecular weight | 164.201 |
| Hydrogen Bond Acceptor | 2 |
| Hydrogen Bond Donor | 0 |
| Polar surface area | 34.601 |
| No. of rotatable bonds | 1 |
| Number of Aromatic Rings | 0 |
| Number of rings | 1 |
| Absorption level | Good |
| Solubility level | Very Soluble |
| Blood Brain Barrier | High |
| Plasma protein binding | Binder |
| CYP2D6 inhibition | Non-Inhibitor |