| ImmunCR Id : | ICR12 |
| Chemical Name : | Dihydroquercetin |
| Plant Source : | Cedrus deodara |
| Nutraceutical information : | Edible, Dihydroquercetin used as efficient antioxidant with regard to vegetable oils, animal fat, milk powder, fat contain pastry |
| Mode of administration : | --- |
| Immunomodulatory mechanism : | Attenutation of IL-6 in brain is symbolic of its anti-neuroinflammatory property, shown to inhibit cerebral ischemia-reperfusion injury in rats through suppression of leukocyte infiltration and by inhibiting clooxygenase-2 and the expression of inducible nitric oxide synthase, attenuated the proteasome inhibition-induced apoptosis in PC12 cells by suppressing the activation of the mitochondrial pathway and aspase-8 and Bid-dependent pathways through antioxidant action. Enhancement in immune status and igm levels Immunostimulant activity for resist and/or treat disease problems |
| Description : | Kadsura, Acacia carneorum, and other species with data are known to contain the natural substance dihydroquercetin. The 2,3-dihydro derivative of quercetin is taxifolin, a pentahydroxyflavanone. |
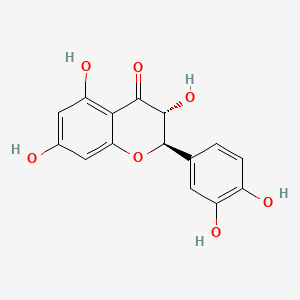
| IUPAC Name | (2R,3R)-2-(3,4-dihydroxyphenyl)-3,5,7-trihydroxy-2,3-dihydrochromen-4-one |
| SMILES | C1=CC(=C(C=C1[C@@H]2[C@H](C(=O)C3=C(C=C(C=C3O2)O)O)O)O)O |
| Formula | C15H12O7 |
| InchiKey | CXQWRCVTCMQVQX-LSDHHAIUSA-N |
| Kingdom | Organic compounds |
| Superclass | Phenylpropanoids and polyketides |
| Class | Flavonoids |
| Subclass | Flavans |
| LogP | 1.479 |
| Molecular weight | 304.252 |
| Hydrogen Bond Acceptor | 7 |
| Hydrogen Bond Donor | 5 |
| Polar surface area | 130.308 |
| No. of rotatable bonds | 1 |
| Number of Aromatic Rings | 2 |
| Number of rings | 3 |
| Absorption level | Moderate |
| Solubility level | Very Soluble |
| Blood Brain Barrier | Undefined |
| Plasma protein binding | Non-Binder |
| CYP2D6 inhibition | Non-Inhibitor |