| ImmunCR Id : | ICR117 |
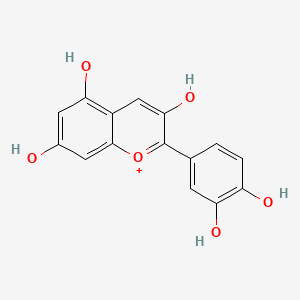
| Chemical Name : | Cyanidin |
| Plant Source : | Vaccinium myrtillus |
| Nutraceutical information : | Edible, Blackberry, cabbage, black grapes |
| Mode of administration : | Oral |
| Immunomodulatory mechanism : | Bone protective (inhibits RANKL (receptor activator of nuclear factor-kb ligand) for osteoclastogenesis in a dose dependent manner via upregulating LXR-β, JAK/STAT signaling, upregulates Sirt6 expression, collagen II protein expression, downregulates ADAMTS5, p38 MAPK, p-HSP27, CXCR4, MMP13, CCR7, IL-17); Antioxidant (inhibits NO, inos); Anti-inflammatory (inhibits COX-2, PGE2, IL-1β, NF-kb p65, TNF-α, iκbα downregulates IL-6); Anti-cancer (upregulates caspase-3 cleavage, G0/G1 phase cell cycle arrest, EGR1, inhibits E-cadherin, downregulates SPEW1, HIF2A, Ki67); Antioxidant (inhibits ROS generation); Anti-apoptosis (downregulates Bax, Nrf2/ARE signaling pathway, caspase-3,-9 expression, ERK phosphorylation, upregulates Bcl-2, inhibits Bak) |
| Description : | Cyanidin is an anthocyanin with potential therapeutic effects. |
| IUPAC Name | 2-(3,4-dihydroxyphenyl)chromenylium-3,5,7-triol |
| SMILES | C1=CC(=C(C=C1C2=[O+]C3=CC(=CC(=C3C=C2O)O)O)O)O |
| Formula | C15H11O6+ |
| InchiKey | VEVZSMAEJFVWIL-UHFFFAOYSA-O |
| Kingdom | Organic compounds |
| Superclass | Phenylpropanoids and polyketides |
| Class | Flavonoids |
| Subclass | Hydroxyflavonoids |
| LogP | 3.038 |
| Molecular weight | 287.244 |
| Hydrogen Bond Acceptor | 5 |
| Hydrogen Bond Donor | 5 |
| Polar surface area | 116.631 |
| No. of rotatable bonds | 1 |
| Number of Aromatic Rings | 3 |
| Number of rings | 3 |
| Absorption level | Good |
| Solubility level | Very Soluble |
| Blood Brain Barrier | Undefined |
| Plasma protein binding | Non-Binder |
| CYP2D6 inhibition | Non-Inhibitor |