| ImmunCR Id : | ICR116 |
| Chemical Name : | Delphinidin |
| Plant Source : | Vaccinium myrtillus |
| Nutraceutical information : | Edible, Grapes, faba beans, peas, hazlenuts, walnuts, lentils (green and red), pomegranate, banana, blueberry. |
| Mode of administration : | Oral |
| Immunomodulatory mechanism : | Anti-cancer (downregulates MAPK (mitogen activated protein kinase), COX-2, Bcl-2, inhibits NF-kb, AP-1, upregulates PARP cleavage, caspase-3,-9,-8, Bax, G2/M phase cell cycle arrest); Anti-inflammatory (inhibits HAT (Histone acetyltransferase), p65 acetylation, TNF-α, upregulates NF-kb); Antioxidant (upregulates p27, HIF-1, P13K/Akt/mtor signaling pathway); Hepatoprotective (upregulates MMP-9, metallothionein I/II); Neuroprotective (downregulates Ca2+, tau phosphorylation, inhibits SIN-1, lipid peroxidation, N-terminal nucleation of SNARE zippering); Anti-angiogenesis (inhibits VEGFR (vascular endothelial growth factor receptor), HIF-1α) |
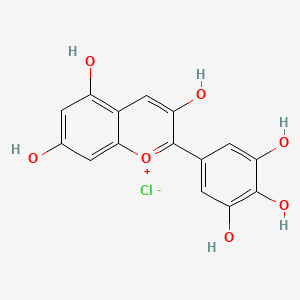
| Description : | Delphinidin, 2-(3, 4, 5-trihydroxyphenyl)chromenylium-3, 5, 7-triol is an anthocynanin diphenylpropane present in the epidermal tissues of fruits, vegetables, and flowers giving them their color and making them useful as a colorant in the food industry. |
| IUPAC Name | 2-(3,4,5-trihydroxyphenyl)chromenylium-3,5,7-triol;chloride |
| SMILES | C1=C(C=C(C(=C1O)O)O)C2=[O+]C3=CC(=CC(=C3C=C2O)O)O.[Cl-] |
| Formula | C15H11ClO7 |
| InchiKey | FFNDMZIBVDSQFI-UHFFFAOYSA-N |
| Kingdom | Organic compounds |
| Superclass | Phenylpropanoids and polyketides |
| Class | Flavonoids |
| Subclass | Hydroxyflavonoids |
| LogP | 0.122 |
| Molecular weight | 338.697 |
| Hydrogen Bond Acceptor | 6 |
| Hydrogen Bond Donor | 6 |
| Polar surface area | 137.447 |
| No. of rotatable bonds | 1 |
| Number of Aromatic Rings | 3 |
| Number of rings | 3 |
| Absorption level | low |
| Solubility level | Very Soluble |
| Blood Brain Barrier | Undefined |
| Plasma protein binding | Non-Binder |
| CYP2D6 inhibition | Non-Inhibitor |