| ImmunCR Id : | ICR114 |
| Chemical Name : | Isoquercitrin |
| Plant Source : | Vaccinium myrtillus |
| Nutraceutical information : | Edible, High content in pistachio nuts, wine, tea, Chinese hawberry, kiwi, chokeberries, |
| Mode of administration : | --- |
| Immunomodulatory mechanism : | Antioxidant (inhibits ROS, RNS, peroxyl , hydroxyl radicals, p47phox protein phophorylation, myeloperoxidase, inos, upregulates GSH, SOD (superoxide dismutase), CAT); Anti-diabetic (inhibit GLUT2); Anti-inflammatory (downregulates PGE2 (prostaglandin E2), IL-6, inhibits COX-2, upregulates Nrf2 xpression); Anti-cancer (inhibits AP-1 (activator protein-1), CYP1B1, CYP1A1, upregulates MMP-9 (matrix metalloproteinases-9), activates Wnt/β-catenin pathway); Neuroprotective (downregulates ages (advanced glycation end products), regulates Rho A gtpase, upregulates SREBP-2 (sterol regulatory element binding protein-2), |
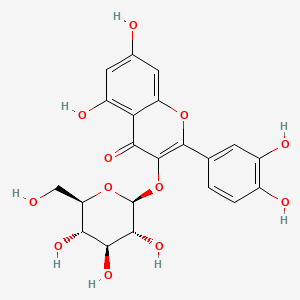
| Description : | Isoquercitrin, quercetin-3-O-β-d-glucopyranoside is a flavanol and a glycosidic form of quercetin, it has two hydroxyl groups on side chains R2, R3. It has poor solubility in water and increases with rise in ph, occurs as a yellow powder. |
| IUPAC Name | 2-(3,4-dihydroxyphenyl)-5,7-dihydroxy-3-[(2S,3R,4S,5S,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]oxychromen-4-one |
| SMILES | C1=CC(=C(C=C1C2=C(C(=O)C3=C(C=C(C=C3O2)O)O)O[C@H]4[C@@H]([C@H]([C@@H]([C@H](O4)CO)O)O)O)O)O |
| Formula | C21H20O12 |
| InchiKey | OVSQVDMCBVZWGM-QSOFNFLRSA-N |
| Kingdom | Organic compounds |
| Superclass | Phenylpropanoids and polyketides |
| Class | Flavonoids |
| Subclass | Flavonoid glycosides |
| LogP | -0.3 |
| Molecular weight | 464.376 |
| Hydrogen Bond Acceptor | 12 |
| Hydrogen Bond Donor | 8 |
| Polar surface area | 210.615 |
| No. of rotatable bonds | 4 |
| Number of Aromatic Rings | 2 |
| Number of rings | 4 |
| Absorption level | Very low |
| Solubility level | Very Soluble |
| Blood Brain Barrier | Undefined |
| Plasma protein binding | Non-Binder |
| CYP2D6 inhibition | Non-Inhibitor |