| ImmunCR Id : | ICR110 |
| Chemical Name : | Allicin |
| Plant Source : | Allium sativum |
| Nutraceutical information : | Edible, Garlic, onion, leek, chives |
| Mode of administration : | Oral |
| Immunomodulatory mechanism : | Cardioprotective (activaes Nrf2/Keap1 pathway signaling, inhibits HMG-coa reductase, acetyl-coa synthase, squalene-monooxygenase, gpiib/iiia, upregulates Nrf2, γ-GCS, NQO1); Anti-cancer (downregulates Bcl-2, Bcl-xl, P13K/mtor pathway signaling, p53, VEGFR2 (vascular endothelial growth factor receptor 2), inhibits HIF-1α, upregulates Nrf2 expression, Bax, Fas, ERK1/2, caspase-3,-8,-9, Beclin-1, AMPK/TSC2 signaling pathway, releases cyctochrome c, ); Antioxidant (inhibits NO, superpoxide radicals, 1,1-diphenyl-2-picrylhydrazyl, hydroxyl free radicals); Anti-inflammatory (inhibits IL-1β, IL-10, MIG, NF-kb, inos, VCAM-1, ICAM-1, downregulates IL-8, IL-1β, iκb) |
| Description : | Allicin, diallylthiosulfinate is a sulfur reactive species, which upon undergoing redox-reaction in protein and glutathione with thiols makes up for allicin biological activity, also releases S-allymercaptoglutathione which is responsible for its antioxidant properties. It makes up a major part of galic defence mechanism. |
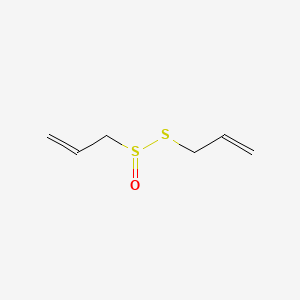
| IUPAC Name | 3-prop-2-enylsulfinylsulfanylprop-1-ene |
| SMILES | C=CCSS(=O)CC=C |
| Formula | C6H10OS2 |
| InchiKey | JDLKFOPOAOFWQN-UHFFFAOYSA-N |
| Kingdom | Organic compounds |
| Superclass | Organic acids and derivatives |
| Class | Thiosulfinic acid esters |
| Subclass | --- |
| LogP | 2.005 |
| Molecular weight | 162.273 |
| Hydrogen Bond Acceptor | 2 |
| Hydrogen Bond Donor | 0 |
| Polar surface area | 17.3 |
| No. of rotatable bonds | 5 |
| Number of Aromatic Rings | 0 |
| Number of rings | 0 |
| Absorption level | Good |
| Solubility level | Very Soluble |
| Blood Brain Barrier | High |
| Plasma protein binding | Non-Binder |
| CYP2D6 inhibition | Non-Inhibitor |