| ImmunCR Id : | ICR106 |
| Chemical Name : | Thymol |
| Plant Source : | Nigella sativa L. |
| Nutraceutical information : | Edible, Kalonji (Black seed) |
| Mode of administration : | Oral, essential oils |
| Immunomodulatory mechanism : | Anti-Alzheimer (inhibits acetylcholinestrase, downregulates amyloid β ); Antibacterial (inhibits biofilm formation, growth, penetrates cell membrane); Anti-fungal (inhibits germination of sporangiospores, mycelia); Antioxidant (inhibits lipid peroxidation, TBARS (Thiobarbituric acid reactive substance), upregulates DPPH (2,2-diphenyl-1-picrylhydrazyl) scavenging, β-carotene, SOD (Superoxide dismutase), Glutathione peroxidase (gpx)); Anti-inflammatory (inhibits TNF-α, IL-6, phosphorylation of MAPK p38, downregulates inos, COX-2); Anti-allergenic (downregulates ige, IL-13, IL-5, IL-4, inhibitss NF-kb pathway); Anti-cancer (G0/G1 phase cell cycle, upregulates ROS, |
| Description : | Thymol, 2-isopropyl-5-methylphenol is a monoterpene phenolic compound mainly found in Lamiaceae family among others. The biosynthesization process of thymol requires aromatization of γ-terpinene to p-cymene and further hyroxylation of p-cymene. It is also used to repel housefly (Drosophila melanogaster), mosquitos, also being used by food industry as a preservative. |
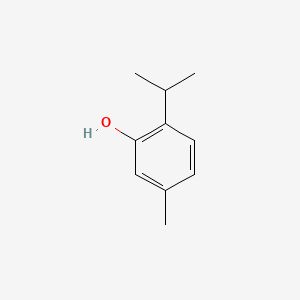
| IUPAC Name | 5-methyl-2-propan-2-ylphenol |
| SMILES | CC1=CC(=C(C=C1)C(C)C)O |
| Formula | C10H14O |
| InchiKey | MGSRCZKZVOBKFT-UHFFFAOYSA-N |
| Kingdom | Organic compounds |
| Superclass | Lipids and lipid-like molecules |
| Class | Prenol lipids |
| Subclass | Monoterpenoids |
| LogP | 3.268 |
| Molecular weight | 150.218 |
| Hydrogen Bond Acceptor | 1 |
| Hydrogen Bond Donor | 1 |
| Polar surface area | 20.815 |
| No. of rotatable bonds | 1 |
| Number of Aromatic Rings | 1 |
| Number of rings | 1 |
| Absorption level | Good |
| Solubility level | Very Soluble |
| Blood Brain Barrier | High |
| Plasma protein binding | Binder |
| CYP2D6 inhibition | Non-Inhibitor |